Food is Information: Nutrigenomics and Nutrigenetics
Scientific knowledge on nutrition and genetics is a newer approach to advancing health outcomes, known as Nutrigenomics. Continue reading to learn how this field can help personalize your dietary needs and improve your health.

Food is information
The concept of “food is information” implies that our food provides us with more than just calories and nutrients. Our food choices and dietary patterns communicate vital information to our bodies that can directly change the course of our health and impact our well-being. The nutrient composition of our meals can inform our bodies to function optimally, help regulate hormones or an anti-inflammatory response in the body, impact the composition of our gut microbiome, act as cellular messengers, and ultimately influence gene expression.
What is nutrigenomics and nutrigenetics?
Nutrigenomics is the study of how nutrients affect your genes. Specifically, it identifies how specific genes can be turned on or off with nutrients and dietary patterns. This helps provide insights into personalized nutrition recommendations based on gene profiling.1,2,3
Nutrigenetics is the study of how your genes respond to nutrients. It explores how genetic variations can impact nutrient utilization from the diet. This helps tailor dietary recommendations to a genetic profile to optimize health.1,2
The fantastic thing about nutritional genomics is that it highlights your influence over your genes! For example, you may have a genetic predisposition for a specific disease, but your environment (including diet and lifestyle) will decide if that gene ever gets “activated” or “turned on.”
How do genes influence how our body utilizes nutrients?
The genes can influence the utilization of nutrients in various ways, including:3,4
- Making enzymes that are essential for food digestion.
- Composition and function of nutrient transporters, responsible for carrying digested nutrients to the bloodstream.
- Regulation of metabolic pathways and nutrient storage and utilization in the body.
- Taste receptors on the tongue for sweet, salty, bitter, and umami tastes influence food choices.
It is important to note that nutrient utilization is a complex interaction between genes and environmental factors such as dietary patterns, lifestyle, and health status.
What are single nucleotide polymorphisms (SNPs)?
Single nucleotide polymorphisms, also known as SNPs (pronounced “snips”), are genetic variations well-established in the population due to mutations and natural selection adaptations in the human genome for survival purposes.3,5,6
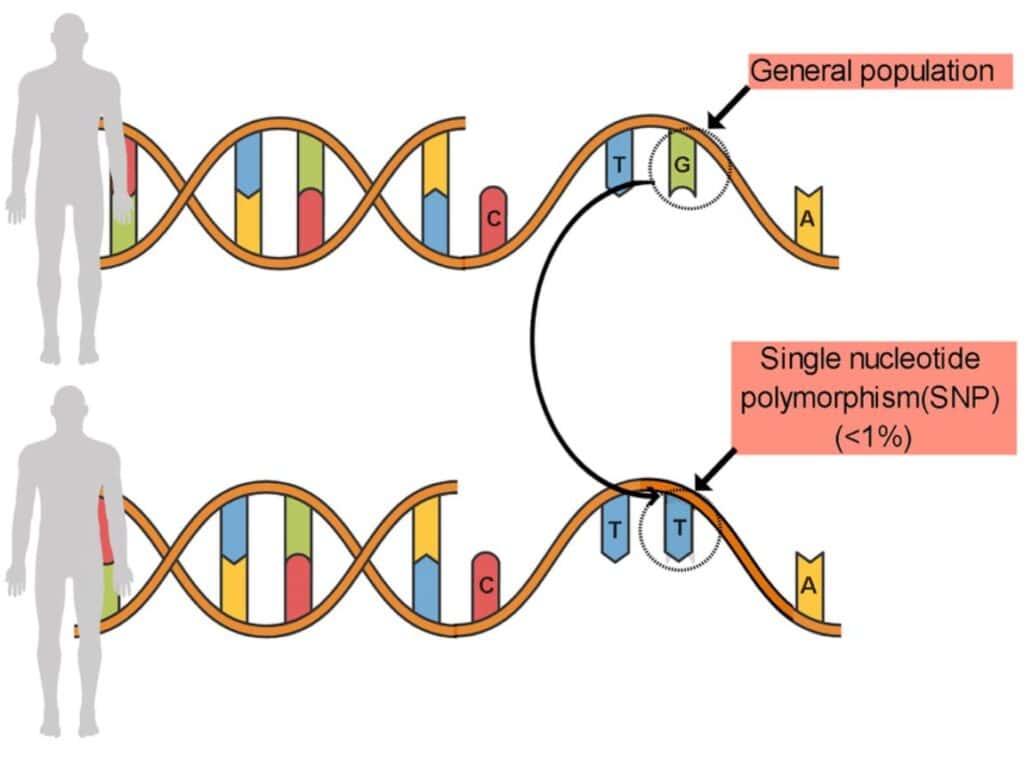
These polymorphisms or SNPs can impact a person’s ability to metabolize, absorb, and utilize nutrients from the diet and increase diet-related diseases or nutrient deficiencies.5,6
Examples of genetic SNPs that may influence how we utilize nutrients:
Genetic polymorphisms are variations in genes that differ between individuals. Here are some examples of SNPs:
- HLA-DQ gene polymorphism increases the likelihood of developing Celiac Disease.3,7
- FTO gene polymorphism is linked to an increased risk of obesity and type 2 diabetes.3,4
- APOE4 gene polymorphism is associated with a predisposition for Alzheimer’s Disease and cardiovascular disease. 3,4,5
- C677T gene polymorphism decreases MTHFR enzyme activity. MTHFR is a key enzyme that regulates folate metabolism and influences levels of the inflammatory marker homocysteine. This polymorphism may increase inflammation in the body and hinder the utilization of folate, which is associated with various diseases, including vascular disease, cancers, diabetes, psoriasis, and may lead to congenital disabilities in babies.4,5,8,9
- TCF7L2 gene polymorphism affects insulin secretion and production, increasing the risk of type 2 diabetes.10,11
- MCM6 gene polymorphism influences the production and activity of lactase enzymes.12,13
SNPs identify genetic variations affecting an individual’s nutritional needs and risks. By understanding your genetic profile, you can decrease the chance of experiencing health issues related to your diet.
How to test for Single Nucleotide Polymorphisms (SNPs)
Many companies offer genetic testing. 23andMe is one popular example. If you choose to do genetic testing or have done it in the past, you can book an appointment to review your results from a nutrition and health standpoint. You can upload your raw genetic data and get this session will give you a comprehensive breakdown of your nutrient-related genetics, SNPs, and actionable steps to prevent or improve your health outcomes. Think of it as an upgrade for your genes. 😀
Book HERE and get 10% off a Nutrigenomics Session!
Take away:
Nutrigenomics and nutrigenetics are fascinating studies of how nutrition and eating patterns impact our genes and how genes can influence our nutrients. Genetic testing and individualized nutritional approaches tailored by registered dietitians help people understand their unique genetic makeup and make informed dietary choices to optimize health.
References:
1. Ordovas, J. M., Mooser, V. (2004). Nutrigenomics and Nutrigenetics. Current Opinion in Lipidology, 15(2): 101-108. https://journals.lww.com/co-lipidology/abstract/2004/04000/nutrigenomics_and_nutrigenetics.2.aspx
2 Asghar, W., & Khalid, N. (2023). Nutrigenetics and nutrigenomics, and precision nutrition. Nutrition and Health. https://doi.org/10.1177/02601060231167228
3 Fenech, M., et al. (2011). Nutrigenetics and Nutrigenomics: Viewpoints on the Current Status and Applications in Nutrition Research and Practice. Journal of Nutrigenetics and Nutrigenomics, 4(2): 69–89. https://karger.com/jnn/article/4/2/69/181452/Nutrigenetics-and-Nutrigenomics-Viewpoints-on-the
4 Barrea, L., Annunziata, G., Bordoni, L., Muscogiuri, G., Colao, A., & Savastano, S. (2020). Nutrigenetics—Personalized nutrition in obesity and cardiovascular diseases. International Journal of Obesity Supplements, 10(1), 1-13. https://doi.org/10.1038/s41367-020-0014-4
5 Nathaniel, M. (2007). Nutrigenomics: The Genome-Food Interface. Environmental Health Perspectives, 115(12): A582-A589. https://ehp.niehs.nih.gov/doi/full/10.1289/ehp.115-a582
6 National Institutes of Health. (2023). Single Nucleotide Polymorphisms (SNPS). https://www.genome.gov/genetics-glossary/Single-Nucleotide-Polymorphisms#:~:text=A%20SNP%20is%20a%20one,people%20and%20even%20between%20populations
7 Iversen, R., & Sollid, L. M. (2023). The Immunobiology and Pathogenesis of Celiac Disease. https://doi.org/10.1146/annurev-pathmechdis-031521-032634
8 Leclerc, D., Sibani, S., Rozen, R. (2013). Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms. Madame Curie Bioscience Database. https://www.ncbi.nlm.nih.gov/books/NBK6561/
9 Liew, S. C., & Gupta, E. D. (2015). Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. European journal of medical genetics, 58(1), 1–10. https://pubmed.ncbi.nlm.nih.gov/25449138/
10 Ciccacci, C., Di Fusco, D., Cacciotti, L. et al. (2013). TCF7L2 Gene Polymorphisms and Type 2 Diabetes: Association with Diabetic Retinopathy and Cardiovascular Autonomic Neuropathy. Acta Diabetol, 50: 789–799. https://link.springer.com/article/10.1007/s00592-012-0418-x
11 Rashid, R., Shah, I. A., Makhdoomi, M.J. et al. (2023). Association of TCF7L2 Gene Variant (rs12255372) with Polycystic Ovary Syndrome and its Effect Modification of the Disease Phenotype. Ind J Clin Biochem. https://doi.org/10.1007/s12291-023-01115-6
12 Robayo-Torres, C., Nichols, B. (2007). Molecular Differentiation of Congenital Lactase Deficiency from Adult-Type Hypolactasia. Nutrition Reviews, 65(2): 95–98. https://academic.oup.com/nutritionreviews/article/65/2/95/1878704
13 Abaturov, A., & Nikulina, A. (2023). Functional annotation of lactase gene and its distal enhancer MCM6 for prediction of metabolically unhealthy obesity. Pediatria Polska – Polish Journal of Paediatrics, 98(1), 16-22. https://doi.org/10.5114/polp.2023.126132
